CSIRO on the frontline for a COVID-19 vaccine
Science and innovation has a massive role to play in fighting the COVID-19 Coronavirus and in solving the challenges of the 21st century. As the national science agency, CSIRO is rising to the challenge.
The COVID-19 Coronavirus pandemic is emerging as the most significant, health, human and economic challenge that Australia and the global community have faced in recent history. As the cases of infection and human toll continues to mount across the world, CSIRO scientists are in the frontline of the global effort to find solutions.
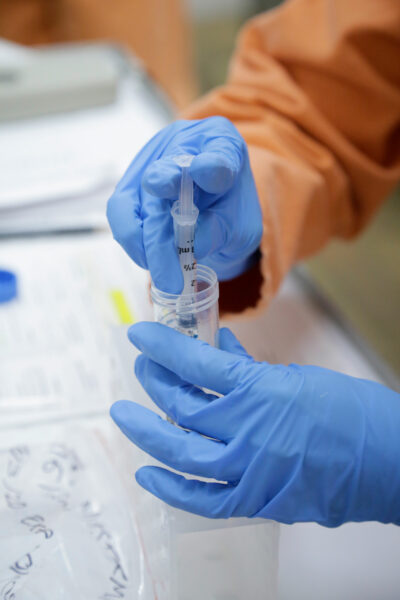
CSIRO has just announced that it is starting the first stage testing of potential vaccines for COVID-19 at CSIRO’s high-containment biosecurity facility, The Australian Centre for Disease Preparedness (ACDP) at Geelong.
CSIRO is testing COVID-19 vaccine candidates for efficacy, but also evaluating the best way to give the vaccine for better protection, including an intra-muscular injection and innovative approaches like a nasal spray.
“Beginning vaccine candidate testing at CSIRO is a critical milestone in the fight against COVID-19, made possible by collaboration both within Australia and across the globe,” according to CSIRO Chief Executive, Dr Larry Marshall.
“CSIRO researchers are working around-the-clock to combat this disease which is affecting so many. Whether it’s at ACDP or at our state-of-the-art biologics manufacturing facility, we will keep working until this viral enemy is defeated,” Dr Marshall said.
The latest milestone builds on CSIRO’s growing work to tackle COVID-19, which has included scaling up other potential vaccine candidates at its biologics production facility in Melbourne.
CSIRO has a long history of developing and testing vaccines since the opening of The Australian Animal Health Laboratory (AAHL) in 1985. The Australian Government announced on April 4 that AAHL was renamed The Australian Centre for Disease Preparedness and would receive a $220 million upgrade. This high containment facility helps protect Australia’s multi-billion dollar livestock and aquaculture industries, and the general public, from emerging infectious disease threats.
To prepare for such outbreaks, last year CSIRO partnered with the Coalition for Epidemic Preparedness Innovations (CEPI), a global group that aims to derail epidemics by speeding up the development of vaccines.
In January, CEPI engaged CSIRO to start working on the virus SARS CoV-2, which causes the disease COVID-19. In consultation with the World Health Organisation, CEPI has identified vaccine candidates from The University of Oxford (UK) and Inovio Pharmaceuticals Inc. (US) to undergo the first pre-clinical trials at CSIRO, with further candidates likely to follow.
CSIRO’s COVID-19 research to date includes:
- being the first research organisation outside of China to generate sufficient stock of the virus to enable pre-clinical studies and research on COVID-19. To do this we used the virus strain isolated by the Doherty Institute.
- successfully establishing a biological model in February 2020, the first in the world to confirm ferrets react to SARS-CoV-2 (the virus that causes COVID-19). Researchers have quickly progressed to studying the course of infection in the animals – a crucial step in understanding if a vaccine will work.
- CSIRO researchers confirmed, after studying SARS CoV-2’s genomic sequence that the virus is presently changing into a number of distinct ‘clusters’ and are now starting to look at how this may also impact on the development of a vaccine.
CSIRO’s COVID-19 work is a key way we are applying science to help solve the greatest challenges.
 |
The Australian Centre for Disease Preparedness is CSIRO’s high-containment biosecurity facility based in Geelong |
 |
Scientists at CSIRO’s ACDP will carry out the first stage testing of potential vaccines for COVID-19 |
 |
 |
